mRNA vaccines and drugs
mRNA vaccines break the immune activation mode of traditional inactivated and attenuated vaccines. They innovatively use the body’s own cells to produce antigens which can activate specific immune response.In addition to being used for the prevention of infectious diseases, the treatment of tumours and immune disorders, mRNAs can also be applied in a variety of biopharmaceutical platform technologies, such as protein supplementation therapies, cellular therapies, antibody-based drugs, gene editing, and other applications. In the post-CoV19 epidemic era, mRNA vaccines still possess a great growth potential.
Preparation of mRNA immune antigen antibodies
mRNA has the potential to encode almost all proteins, and protein antigens and antibodies can theoretically be produced using mRNA technology. mRNA vaccine technology can be applied to antigen and antibody preparation, and used to solve the problems of protein and antibody preparation such as :long lead time of traditional protein antigens, complicated production process and technology, and less flexibility in production customization. As a platform technology, mRNA has the advantages of simple production process, short production cycle, flexibility, etc. It has the potential to encode almost all proteins, and can provide customers with rapid target feasibility verification in a faster and more cost-effective way.
· Hzymes biotech provides One-Stop mRNA vaccine&drug Proof of concept (POC) services to help clients rapidly complete the feasibility study of mRNA drug and vaccine.
- Experienced team:successfully delivered multiple proof-of-concept projects
- Diversified tools:utilizing alphafold to predict the secondary structure of proteins, to provide guidance for customers' antigen design and mRNA sequence design.
- Self-established mRNA technology and application platform: perfect quality system, full-process service from sequence design, mRNA-LNP preparation to in vivo biological evaluation.








Service items | Periodicity (Working days) | Deliverables |
Sequence design | 5 | 10 candidate sequences |
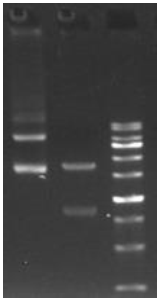
Plasmid construction and amplification | 30 | Provide synthesised original plasmid (1-2 μg), with its sequence files |
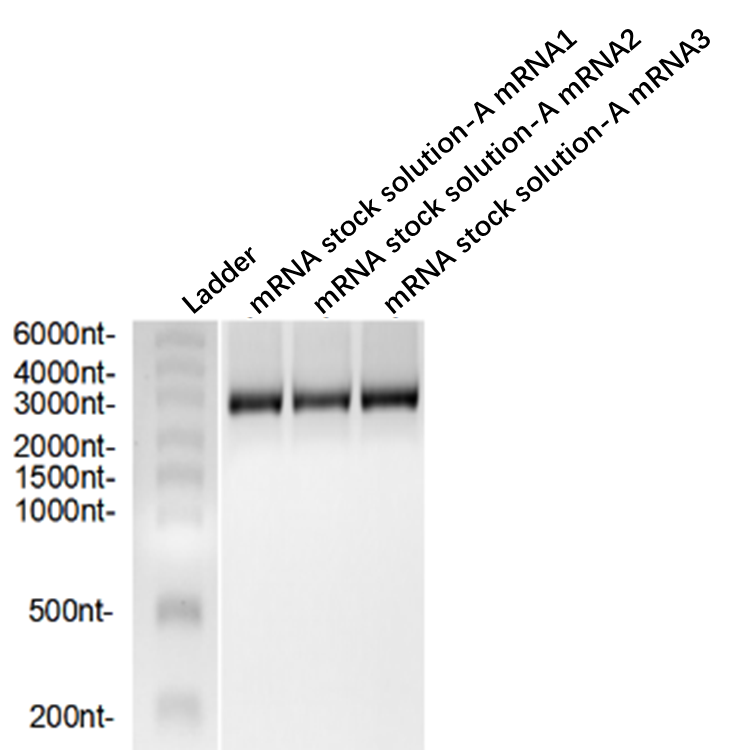
mRNA stock solution synthesis | 5 | Provide a test report for mRNA stock solution |
Detection of mRNA translation levels in cells | 10 | Provide a test report for mRNA expression levels in cells |
LNP encapsulation | 10 | 1mg LNP |
In vivo expression verification (optional) | 20 | Test report |
In vivo assessment of Cellular immunity (optional) | 10 | Test report |
In vivo assessment of humoral immunity (optional) | 20 | Test report |
In vivo expression verification+Cellular immunity (optional) | 20 | Test report |
In vivo expression verification+Cellular immunity +humoral immunity (optional) | 20 | Test report |
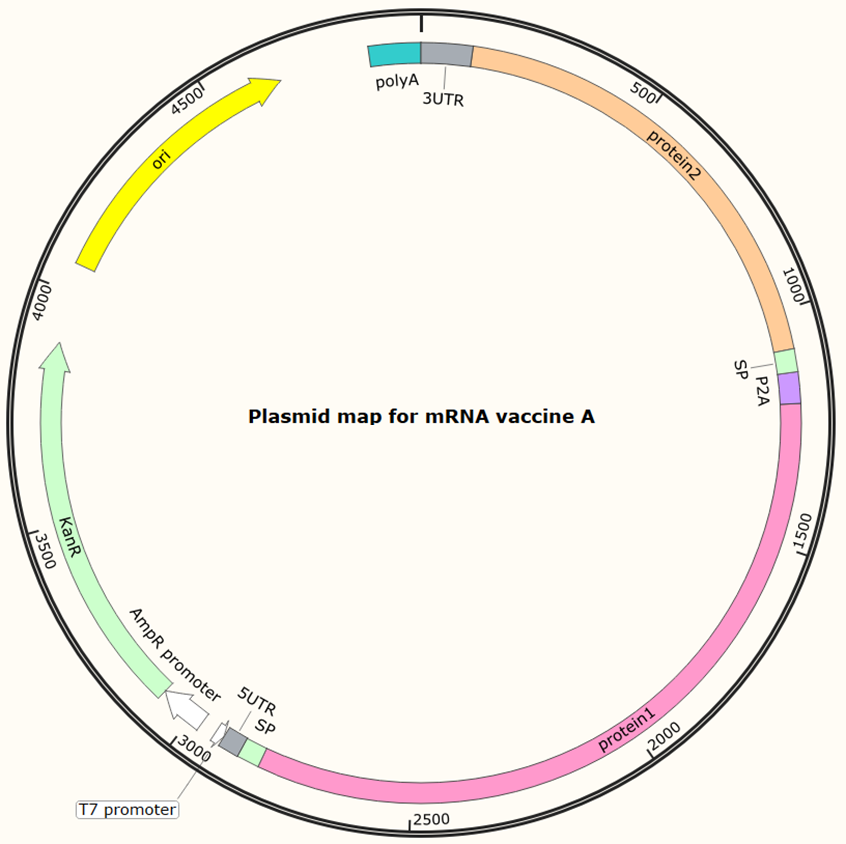



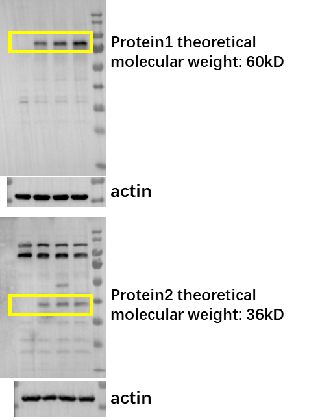
| No. | mRNA | integrity |
| 1 | A mRNA1 | 84% |
| 2 | A mRNA2 | 85% |
| 3 | A mRNA3 | 85% |
| Sample | Size:nm | PDI | Encapsulation rate |
| A mRNA1-LNP | 86.63 | 0.09 | 96.50% |
| A mRNA2-LNP | 87.32 | 0.07 | 97.28% |
| A mRNA3-LNP | 85.09 | 0.1 | 98.17% |