IVT (in vitro transcription), which is for mRNA synthesis, is the core part of the whole mRNA preparation process. In addition to the synthetic modification of mRNA, how to deliver mRNA safely and effectively into the body is also the highlight and difficulty of mRNA technology development. For mRNA vaccines and drugs, the delivery system has three major responsibilities:effectively wrapping and protecting the mRNA to maintain stability before reaching the target, helping the mRNA active ingredient to enter the cell, and releasing the mRNA into the cytoplasm before it reaches the lysosome. Lipid nanoparticle LNP is one of the best choices for mRNA vaccine delivery when all other carriers have a distinct disadvantage.
■ Hzymes Biotech provides the materials such as restriction endonucleases for plasmid templates linearization, enzymes for in vitro transcription, modified nucleosides, cap analogues, and materials for purification and quality control,provides custom synthesis of mRNA and mRNA-LNP in different specifications with high yield, high integrity and high cost-effectiveness.
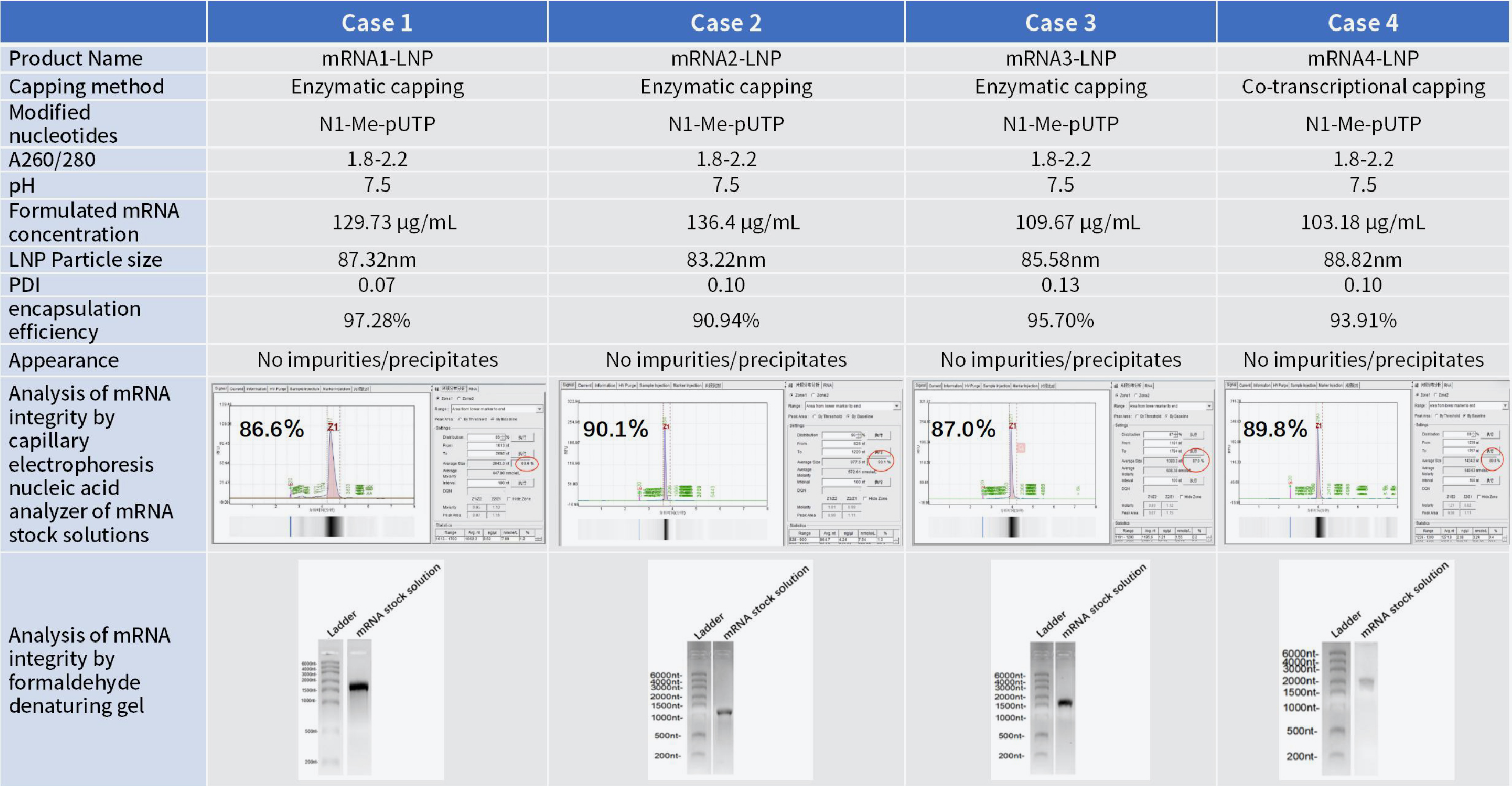
■ Hzymes Biotech adopted a concise and rapid microfluidic technique for the preparation of mRNA-LNP, which is highly stable as well as easy to produce and scale up. After the encapsulation process, naked mRNA was finally enveloped in LNP, which effectively protects the mRNA and transports it into the cell to perform its function.
■ Hzymes Biotech provide one-stop complete solution, customers can choose customized production at any step from sequence design to LNP preparation, get the required mRNA stock and LNP stock more quickly and efficiently to the greatest extent possible.









Service classes | Items | Periodicity (Working days) | Deliverables |
Sequence Design (not undertaken separately) | Sequence design | 5 | Sequence files |
Template preparation (not undertaken separately) | Plasmid synthesis | 20 | Provide sequence files of plasmid. Provide synthesised original plasmid (1-2 μg) if required by client |
Plasmid amplification | 5 | Plasmid sequencing files, if requested by the customer, the production of surplus plasmids can be provided to the customer, no surplus plasmids do not do additional preparations | |
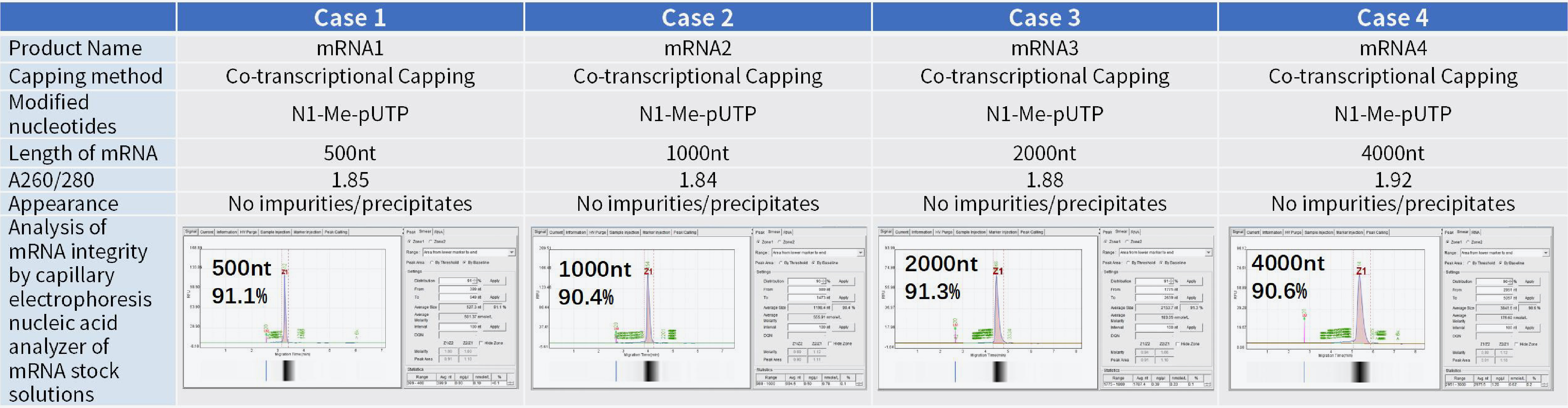
mRNA stock solution synthesis | mRNA stock synthesis - unmodified mRNA (research grade) | 5 | mRNA stock solution and CoA |
mRNA stock synthesis - nucleotide modified mRNA (research grade) | 5 | ||
mRNA stock synthesis - unmodified mRNA (industrial grade) | 5 | ||
mRNA stock synthesis - nucleotide modified mRNA (industrial grade) | 5 | ||
Rapid synthesis of mRNA stock solution (Plasmid rapid synthesis to stock solution; rapid synthesis packaging service) | Rapid synthesis of mRNA stock solutions (research grade) | 20 | mRNA stock solution and CoA |
LNP | Encapsulation only | 5 | LNP and CoA |
mRNA synthesis and LNP encapsulation(research grade) | 13 | ||
mRNA synthesis and LNP encapsulation (industrial grade) | 13 |
Service classes | Items | Periodicity (Working days) | Test method |
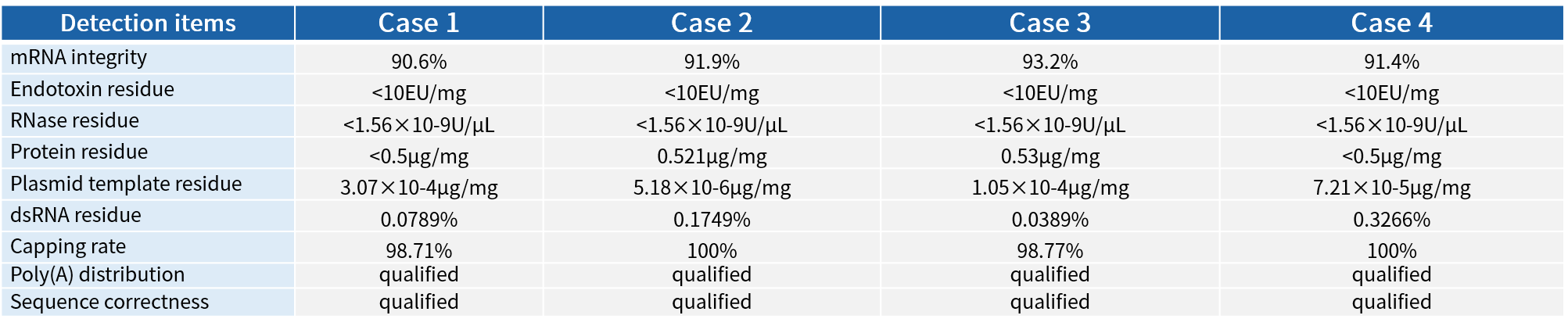
Quality control services (not undertaken separately) | dsRNA | 1 | ELISA |
Total protein residues | 1 | Nano Orange | |
DNA template residue | 1 | Quantitative Real-time PCR | |
endotoxin | 1 | LAL | |
RNase residue | 1 | Fluorescence probe method | |
DNase residue | 1 | Fluorescence probe method | |
Host DNA residue | 1 | Quantitative Real-time PCR | |
NTP and cap analogue residue | 1 | HPLC |